Electrolyte in Solid State Battery (SSB), which ships first?
- Laras Fadillah
- Sep 29, 2025
- 2 min read
Updated: Sep 30, 2025
When people imagine solid-state batteries, they picture a "perfect electrolyte" that fixes safety, boosts energy, and charges fast. In practice, every family (sulfides, oxides, polymers) wins on some axes and loses on others. The first to ship at scale won't be the most "perfect" but the one that balances performance + manufacturability + safety well enough to leave the lab.
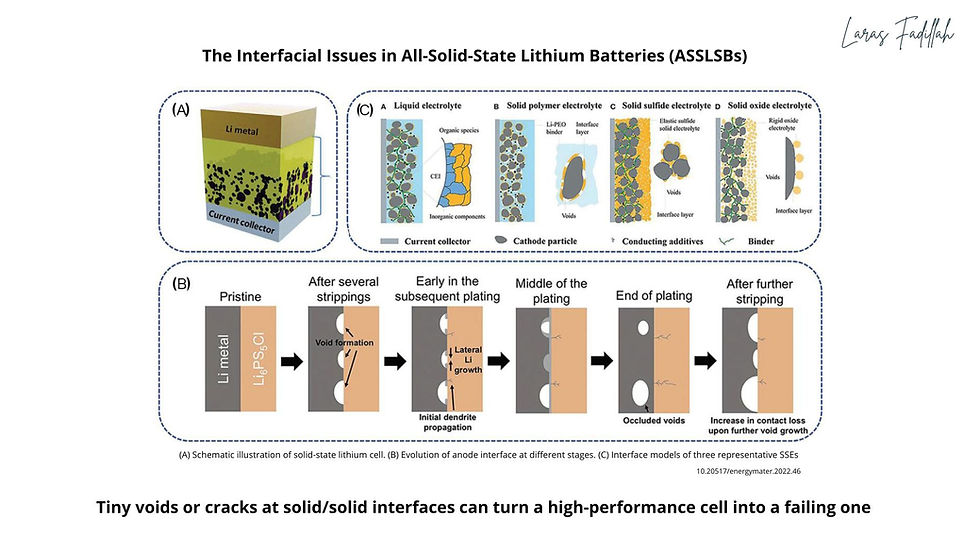
- Sulfides feel closest to liquid electrolytes in speed. They routinely post liquid-like ionic conductivities, and their ductility helps form intimate contact. But air is their nemesis: moisture can trigger decomposition (including H₂S release), which clashes with today's Li-ion production lines. A recent study showed a clever surface-molecular "raincoat" that lets Li₆PS₅Cl be processed under humid ambient air - a real step toward factory compatibility, though not a full solve for long-term interfacial chemistry.
- Oxides (think garnets like LLZO) shine on stability. They're non-toxic, handle high voltages, and don't mind air the way sulfides do. The flip side is ceramic reality: brittleness, lower room-temperature conductivity vs sulfides, and high-temperature densification to eliminate porosity. New sintering routes (field-assisted/joule heating) are improving densification and grain-boundary quality, but they add process complexity industry must absorb.
- Polymers are the pragmatic middle path. They're mechanically forgiving, can be laminated into thin films, and play nicely with roll-to-roll. The historical knock is room-temperature conductivity, often requiring elevated temperature or composite tricks. Yet polymers are the first SSBs you can actually buy in meaningful products. France's Blue Solutions (Bolloré) already supplies solid-polymer batteries for buses and is building a €2B SSB gigafactory targeting EV-class cells with 30–40% higher energy density than today’s top Li-ion and sub-20-minute charging, in partnership with automakers like BMW (ramping to 25 GWh by ~2030).
- Meanwhile, semi-solid/hybrid routes (pairing polymer/gel with solid or high-loading cathodes) are bridging to market. WeLion's packs in NIO demonstrations (150 kWh, long-range drives) show how "good-enough" solid/gel architectures can deliver real-world range while more brittle chemistries mature. These are not the endgame, but they are shipping signals.
So what actually reaches broad adoption first?

The "winner" is the chemistry that builds a supply chain and passes abuse tests before rivals do. Right now, that advantage leans polymer/hybrid, while sulfide and oxide lines push hard on process and interface breakthroughs. Their systems in wearables, mobility fleets, and early automotive programs, because factories can laminate them, OEMs can qualify safety faster, and the supply chain exists
Comments