Solid-State Battery Interfaces: The Hidden Challenge to Solve
- Laras Fadillah
- Sep 30, 2025
- 2 min read
Solid-state batteries promise so much: higher safety, higher energy density, cleaner designs. But there's a catch: the interfaces. Where two solids meet, problems emerge, and in ASSLSBs, they're often the root cause of failure.
I remember reading Interfacial Challenges in All-Solid-State Lithium Batteries (Huang, 2022) and thinking: “Yes, this is the crux.” They outline how solid/solid interfaces suffer from poor contact, chemical mismatches, and mechanical stress. Even tiny voids or pores between the solid electrolyte and electrode are enough to drastically raise internal resistance.
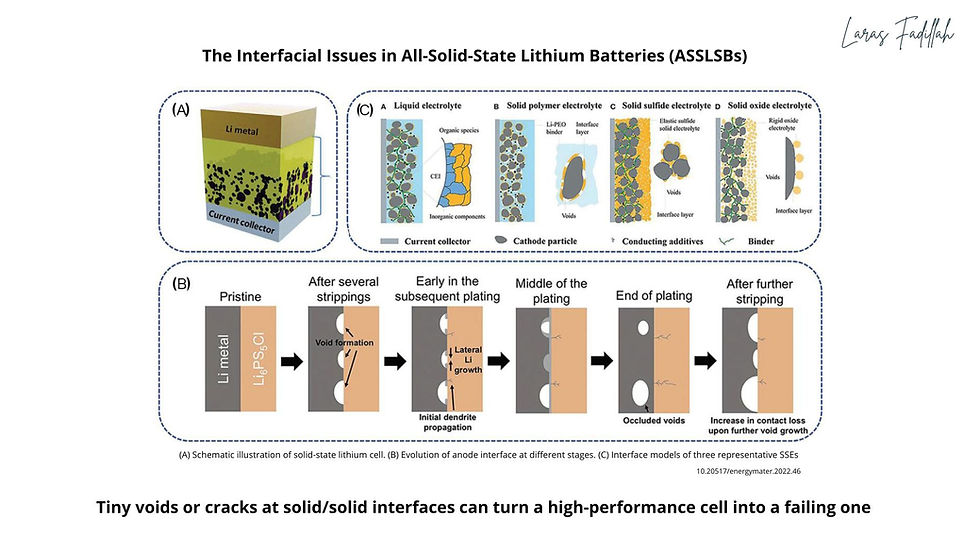
Joshi et al. (2025) highlight how solid-state battery interface problems evolve during cycling: electrodes expand and contract, creating cracks; lithium plating generates uneven layers; resistive interphases grow at contact points. Maintaining contact often requires external stack pressure or advanced densification. https://advanced.onlinelibrary.wiley.com/doi/10.1002/adma.202000721
A few well-studied examples:
- Chemical instability: sulfide electrolytes react with high-voltage cathodes, forming side products that block ion transport.
- Mechanical stress: electrodes expand and contract during cycling, but rigid electrolytes cannot "flow" to accommodate, leading to cracks and delamination.
- Lithium metal: voids form during stripping, and when lithium re-deposits, dendrites propagate through the gaps, a failure pathway mapped in detail here: https://www.frontiersin.org/journals/chemistry/articles/10.3389/fchem.2018.00616/full
Researchers are testing multiple solutions: protective coatings, engineered interlayers, composite cathodes with elastic binders, or even electrolyte designs with built-in flexibility. Each strategy provides some stability, but none has yet solved the full complexity.
And this is the heart of the challenge: the electrolyte material might have world-class conductivity, or the anode might promise record capacity, but if the interface fails, the whole cell fails. The invisible line where two solids touch becomes the ultimate bottleneck.
Even world-class materials fail if their interfaces can't stay in contact.

Comments